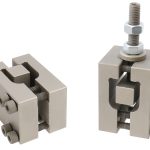
ASTM F2077 Test Fikstürü
ASTM F2077 covers the materials and methods for the static and dynamic testing of intervertebral body fusion device assemblies, spinal implants designed to promote
arthrodesis at a given spinal motion segment.
Bilgi Almak İçin Lütfen Bizimle İletişime Geçiniz
- Açıklama
- Değerlendirmeler (0)
- TEKNİK ÖZELLİKLER
Açıklama
ASTM F2077 – Standard Test Methods For Intervertebral Body Fusion Device
ASTM F2077 covers the materials and methods for the static and dynamic testing of intervertebral body fusion device assemblies, spinal implants designed to
promote arthrodesis at a given spinal motion segment.
This test method is intended to provide a basis for the mechanical comparison among past, present, and future nonbiologic intervertebral body fusion device
assemblies.
This test method allows comparison of intervertebral body fusion device assemblies with different intended spinal locations and methods of application to
the intradiscal spaces.
This test method is intended to enable the user to compare intervertebral body fusion device assemblies mechanically and does not purport to provide performance
standards for intervertebral body fusion device assemblies.
The test method describes static and dynamic tests by specifying force types and specific methods of applying these forces.
These tests are designed to allow for the comparative evaluation of intervertebral body fusion device assemblies.
These tests are designed to characterize the structural integrity of the device and are not intended to test the bone-implant interface.
Guidelines are established for measuring displacements, determining the yield force or moment, and evaluating the stiffness,stiffness and strength of the
intervertebral body fusion device assemblies.
Some intervertebral body fusion device assemblies may not be testable in all test configurations.
ASTM F2077 / Significance and Use
Intervertebral body fusion device assemblies are generally simple geometric-shaped devices which are often porous or hollow in nature.
Their function is to support the anterior column of the spine to facilitate arthrodesis of the motion segment.
This test method outlines materials and methods for the characterization and evaluation of the mechanical performance of different intervertebral body fusion
device assemblies so that comparisons can be made between different designs.
This test method is designed to quantify the static and dynamic characteristics of different designs of intervertebral body Fusion device assemblies.
These tests are conducted in vitro to allow for analysis and comparison of the mechanical performance of intervertebral body fusion device assemblies to
specific force modalities.
The forces applied to the intervertebral body fusion assemblies may differ from the complex loading seen in vivo, and therefore, the results from these tests may not
directly predict in vivo performance.
The results, however, can be used to compare mechanical performance of different intervertebral body fusion device assemblies.
Since the environment may affect the dynamic performance of intervertebral body fusion device assemblies, dynamic testing in a saline environment may be
considered.
Fatigue tests should first be conducted in air (at ambient temperature) for comparison purposes since the environmental effects could be significant.
If a simulated in vivo environment is desired, the investigator should consider testing in a saline environmental bath at 37°C37 °C (for example, 0.9-g 0.9 g NaCl per
100-mL100 mL water) at a rate of 1 Hz or less.
A simulated body fluid, a saline drip or mist, distilled water, or other type of lubrication at 37°C37 °C could also be used with adequate justification.
If the devices are known to be temperature and environment dependent.
Devices that require physiologic solution for testing should be tested in the same type solution for comparison purposes.
The location within the simulated vertebral bodies and position of the intervertebral body fusion device assembly with respect to the loading axis will be dependent
upon the design, the manufacturer’s recommendation, or the surgeon’s preferred method for implant placement.
It is well known that the failure of materials is dependent upon stress, test frequency, surface treatments, and environmental factors.
Therefore, when determining the effect of changing one of these parameters (for example, frequency, material, or environment), all others must be kept constant
to facilitate interpretation of the results.
ASTM F2077 / Apparatus
Test machines will conform to the requirements of Practices ASTM E4.
The intradiscal height, H, shall be determined from vertebral body and disc morphometric data at the intended level of application.
Suggested heights are as follows: 10 mm for the lumbar spine, 6 mm for the thoracic spine, and 4 mm for the cervical spine.
The intradiscal height should not reach zero before the onset of functional or mechanical failure. If this occurs, the test is considered a failure.
The user of the test method should select the intradiscal height that is appropriate for the device being tested.
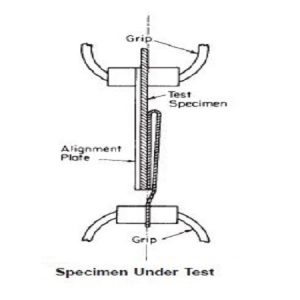
ASTM F2077 / Axial Compression Test Apparatus
The actuator of the testing machine is connected to the pushrod by a minimal friction ball and socket ball-and-socket joint or universal joint
(that is, unconstrained in bending).
The pushrod is connected to the superior fixture by a minimal friction sphere joint (that is, unconstrained in bending and torsion).
The hollow pushrod should be of minimal weight so as to be considered a “two-force” member. It thus applies to the intervertebral body fusion device assembly
a resultant force directed along the pushrod’s axis and located at the center of the superior fixture’s sphere joint (the geometric center of the device being tested).
For the fatigue tests, the device is placed between two polyacetal blocks, which are rigidly attached to the metal blocks.
For the static tests, metal blocks are to be used, which could be incorporated as an integral part of the superior and inferior fixtures.
The blocks are to have surfaces that mate geometrically with the intervertebral device similar to how the device is intended to mate with vertebral end plates.
The test apparatus will be assembled such that the Z axis of the intervertebral device is initially coincident with the pushrod’s axis and collinear with the axis of the
testing machine’s actuator and load cell.
The length of the pushrod between the center of the ball-and-socket joint to the center of the spherical surface is to be a minimum of 38 cm.
This is required to minimize deviation of the pushrod’s axis (direction of applied force, F) from that of the test machine’s load cell axis.
In other words, this is to minimize the error in using and reporting that the force indicated by the load cell “Find” is the applied force, F, and is equal to
the compressive force, Fz, on the intervertebral body fusion device assembly.
For example, a 1-mm displacement of the spherical surfaces center in the XY plane would produce an angle between axes of 0.15°, (10 mm
producing 1.5°).
ASTM F2077 / Compression-Shear Testing Apparatus—The compression-shear test apparatus , with exception of the inferior fixture, is identical to the axial
compression apparatus .
The inferior fixture is to be designed to orient the initial position of the intervertebral device’s Z axis at either 45° or 27°3 flexion relative to the pushrod’s axis.
The resultant force, F, being applied to the intervertebral body fusion device assembly passes through the center of the superior fixture’s spherical surface and
is coincident with the pushrod’s axis.
Thus, a combined compressive force Fz and an anterior shear force Fx is created, which initially are either equal in magnitude or Fz is twice that of Fx and passes
through the geometric center of the intervertebral body fusion device assembly.
*** Before conducting ASTM F2077 , it is important to read the entire specification. Standards can be obtained from appropriate standard authorities.
***PARSROS offers several types of grips and fixtures which will enable you to perform a variety of tests
that are accurate and repeatable.




























Bir yanıt yazın
Yorum yapabilmek için oturum açmalısınız.