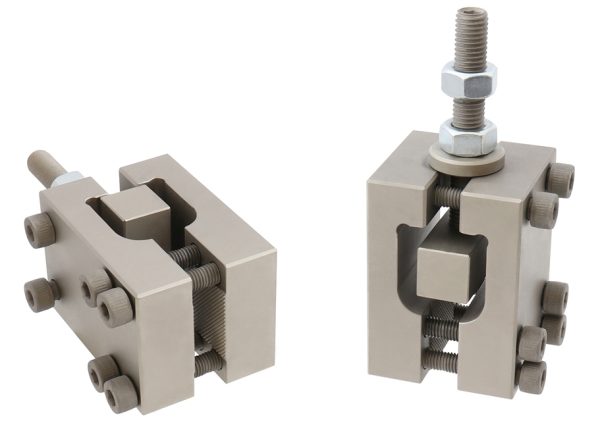
ASTM F2118 Test Fikstürü
ASTM F2118 describes test procedures for evaluating the constant amplitude, uniaxial, tension-compression uniform fatigue performance of acrylic bone
cement materials.
Bilgi Almak İçin Lütfen Bizimle İletişime Geçiniz
- Açıklama
- Değerlendirmeler (0)
- TEKNİK ÖZELLİKLER
Açıklama
Açıklama
ASTM F2118 – Standard Test Method for Constant Amplitude of Force Controlled Fatigue Testing of Acrylic Bone Cement Materials
ASTM F2118 describes test procedures for evaluating the constant amplitude, uniaxial, tension-compression uniform fatigue performance of acrylic bone
cement materials.
This test method is relevant to orthopedic bone cements based on acrylic resins, as specified in Specification ASTM F451 and ISO 16402.
The procedures in this test method may or may not apply to other surgical cement materials.
It is not the intention of this test method to define levels of performance of these materials.
It is not the intention of this test method to directly simulate the clinical use of these materials, but rather to allow for comparison between acrylic bone cements to
evaluate fatigue behavior under specified
ASTM F2118 / Significance and Use
This test method describes a uniaxial, constant amplitude, fully reversed fatigue test to characterize the fatigue performance of a uniform cylindrical waisted
specimen manufactured from acrylic bone cement.
This test method considers two approaches to evaluating the fatigue performance of bone cement:
Testing is conducted at three stress levels to characterize the general fatigue behavior of a cement over a range of stresses.
The stress level and resultant cycles to failure of the specimens can plotted on an S-N diagram.
Another approach is to determine the fatigue life of a particular cement.
The fatigue life for orthopaedic bone cement is to be determined up to 5 million (5 × 106) cycles.
This test method does not define or suggest required levels of performance of bone cement.
This fatigue test method is not intended to represent the clinical use of orthopaedic bone cement, but rather to characterize the material using standard and
well-established methods.
The user is cautioned to consider the appropriateness of this test method in view of the material being tested and its potential application.
It is widely reported that multiple clinical factors affect the fatigue performance of orthopaedic bone cement; however, the actual mechanisms involves multiple
factors.
Clinical factors which may affect the performance of bone cement include: temperature and humidity, mixing method, time of application, surgical technique,
bone preparation, implant design, anatomical site, and patient factors, among others.
This test method does not specifically address all of these clinical factors.
The test method can be used to compare different acrylic bone cement formulations and products and different mixing methods and environments (that is, mixing
temperature, vacuum, centrifugation, and so forth). conditions.
ASTM F2118 / Apparatus
ASTM F2118 / Uniaxial Load Frame—A testing machine capable of applying cyclic sinusoidal tensile and compressive loads.
The crossheads of the load frame shall be aligned such that the alignment meets the requirements Practice E466.
The alignment should be checked at both the maximum tensile and minimum compressive load to be applied during the course of a test program.
Cycle Counter—A device capable of counting the number of loading cycles applied to a specimen during the course of a fatigue test.
Load Cell—A load cell capable of measuring dynamic tensile and compressive loads in accordance with Practice ASTM E467.
Limit—A device capable of detecting when a test parameter (for example, load magnitude, actuator displacement, DC error, and so forth) reaches a limiting value,
at which time the test is stopped and the current cycle count recorded.
ASTM F2118 / Environmental Chamber—A chamber designed to immerse the fatigue specimen completely in a solution.
The chamber should have provisions for maintaining a constant temperature to an accuracy of 62°C.
ASTM F2118 / Test Specimen
Test specimens shall be fabricated from cement that is representative of the final product with regard to materials, manufacturing processes, sterilization, and
packaging.
Certain sterilization methods have been shown to have an effect on fatigue performance (for example, gamma sterilization of the powder).
Any deviations of the test cement from the clinically used product must be reported.
Cylindrical reduced gage section test specimens with a straight 5-mm diameter by 10-mm-long gage section shall be used.
The diameter of the specimen ends shall be substantially greater than the gage diameter to ensure that fracture occurs in the gage section.
A smooth surface of the test specimen in the radius or taper between the specimen ends and gage section is essential to reduce variation in reported fatigue life.
*** Before conducting ASTM F2118 , it is important to read the entire specification. Standards can be obtained from appropriate standard authorities.
***PARSROS offers several types of grips and fixtures which will enable you to perform a variety of tests
that are accurate and repeatable.























Bir yanıt yazın